Chapter 1: What Is a Transdermal Patch?
Transdermal Medication Patch 101
Transdermal patches are a method of drug delivery in which an adhesive patch provides a pre-prescribed dose of medication that is absorbed through the skin and into the bloodstream. The patches provide a non-invasive method of drug delivery while allowing for a steady absorption of a medication over an extended period of time, providing an alternative delivery method to the immediacy of an injection or liquid medication.
While topical medication can be traced back as far as ancient China, transdermal patches are relatively new to the market. The first FDA-approved patch appeared in 1979 to treat motion sickness, and patches expanded further with the approval of the Nicotine patch in the 1990s. Today, patches are commonly used to treat Alzheimer's disease and Parkinson's and administer birth control.
Depending on the dosage and type of medication, patches on the market today can be worn anywhere between seven hours and eight days. With minimal inconvenience to patients, and the reassurance of more consistent dosages for prescribers, transdermal patches are quickly becoming the preferred drug delivery system.
How to Make a Transdermal Patch
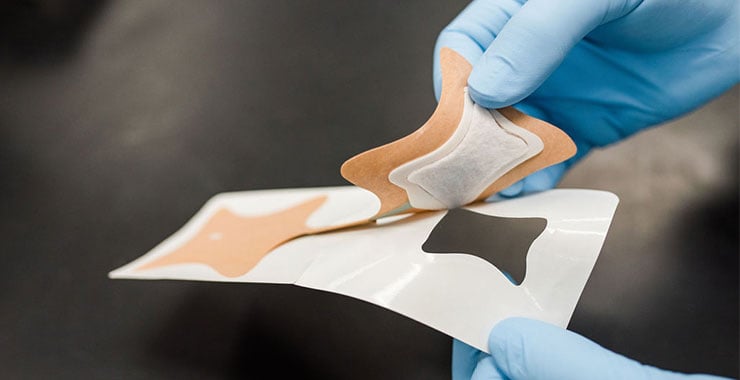
Each transdermal medication patch will differ depending on the drug being administered, but all patches are composed of the same basic elements:
- Backing Layer: The most visible section, this protects the patch from the environment and contaminates while it's adhered to a patient's skin. Drug Layer/Reservoir: The medication contained in the patch. Depending on the type of patch, this can either be a separate layer or part of the adhesive layer.
- Adhesive: The sticking layer which adheres the patch to the skin, and in some patches releases the drug. (Note that adhesives for transdermal patches must be skin-safe, medical grade, and heat-activated.)
- Liner: The covering for the adhesive prior to use. This is removed before applying the patch.
Some patches also include an enhancer to increase delivery rate, stabilizers (antioxidants), or preservatives.
Types of Transdermal Patches
There are five common types of transdermal patches:
- Single-Layer Drug-in-Adhesive: The entire patch is composed of a single layer of adhesive which includes the drug dosage. When applied, this layer both sticks to the skin and releases the drug simultaneously.
- Multi-Layer Drug-in-Adhesive: Similar to the single-layer, but contains more than one layer of drug-in-adhesive. Usually used for longer-term patches. Each layer will start diffusion through the next as the layers closest to the skin finish delivering the drug.
- Reservoir: The drugs in a reservoir patch are stored in their own liquid layer, separate from the adhesive. When applied to the skin, the drug slowly leaves the liquid layer through a rate-controlling membrane. The drugs then move through the adhesive to the skin. A top layer contains the other side of the liquid drug layer.
- Matrix: The drugs are kept in a semi-solid layer, referred to as a matrix. The adhesive layer in this type of patch usually surrounds this matrix, similar to a reservoir path.
- Vapor Patch: The adhesive layer of a vapor patch also serves as a vector for releasing vapor. Vapor patches can last up to six hours and are most commonly used as decongestants or sleep aids.
All five of these methods work through either a passive or active delivery system.
Passive systems are known for their consistency. They rely on natural membrane diffusion to transfer the drug from the patch, through the skin, and into the blood.
Active systems use an enhancer or additional aid to transfer the drug. Sometimes these are enhancers within a patch, but they can also include alternative transdermal medications.
Transdermal Patch vs. Other Transdermal Medications
The transdermal patch is the most popular delivery method amongst transdermal medications; however, there are other transdermal methods available — the most popular being microneedles and transdermal gel.
Microneedles are short, thin needles coated in a prescribed medication. When they pierce the skin, they increase permeability and inject the drug closer to the bloodstream so that it can be more easily absorbed.
Transdermal gels are rubbed into the skin in prescribed doses. These are usually over-the-counter medications such as topical ointments or pain relief.
Chapter 2: How a Transdermal Patch Works
Transdermal Patch Efficacy
While transdermal patches are not ideal for every medication, they have proven successful for small-molecule medications. They have also significantly improved medication adherence and delivery for patients with strong sensitivities to injections or oral medications.
Depending on the medication, some patches can also provide a higher and/or more effective dose of a medication as it is released over time. Through the use of patches, medicine can also be directed to a specific area of pain rather than a general body pain reliever.
Additionally, transdermal patches show promise for future medications as scientists close in on new ways to adapt patches for large-molecule drug delivery.
Benefits of Transdermal Patches
Transdermal patches provide a number of advantages over pill, powder, or liquid medications.
- Transdermal patches deliver medication more directly to the bloodstream by bypassing processing in the liver.
- Transdermal patches deliver medication over a longer period of time than traditional systems, which can be easier on patients who experience extreme side effects.
- Transdermal patches bypass the digestive system, minimizing the impact of corrosive stomach acid on the medication.
- Transdermal patches are applicable by the patient, which eliminates multiple trips to a doctor's office. This also makes them convenient to use.
- Transdermal and topical medications carry a lower risk of medication addiction.
- Transdermal patches eliminate the need for injections, often making them more comfortable for patients and improving medication adherence.
What Types of Drugs are Administered Through Transdermal Patches?
The most widely used transdermal patch is the nicotine patch, which delivers nicotine to the user in an effort to curb the urge to smoke tobacco.
Other common transdermal patches include hormonal patches (estrogen, testosterone, and contraception), vitamin supplement patches, pain relief patches (fentanyl and buprenorphine), and motion sickness patches (scopolamine).
Transdermal patches have also been created for numerous additional drugs; however, they are alternative versions of a pill or liquid drug delivery system, not the primary system (i.e. antidepressants).
Applying Transdermal Patches
The majority of patches will follow a similar application process:
- Wash hands and the area of skin where the patch will go with soap and water.
- Remove the liner from the patch to expose the adhesive (similar to a Band-Aid).
- Place the patch on the cleaned area of skin with the adhesive facing downward.
- Press down on the patch and smooth out any lumps in the adhesive. This will ensure the patch is secure, and in some cases start the flow of medication.
- Remove any remaining packaging from the patch.
- Wash your hands once again to make sure you are not accidentally spreading the medication to a sensitive part of your body, such as your eyes.
Transdermal Patch Side Effects
All drugs and drug delivery systems come with the potential for side effects. Side effects for transdermal patches will vary depending on the medication; there are also possible side effects from the patch itself.
Just as nausea is the most common side effect of an oral drug delivery system, irritated skin is the most common side effect for the transdermal patch. This irritation can be caused by the adhesive of the patch or the medication it's distributing.
If these signs of irritation appear, patients are encouraged to consult their doctor or prescriber immediately.
Learn More about how transdermal patches work.
Chapter 3: Transdermal Development and Manufacturing
The Drug Development Process
Transdermal patches must follow the same development and manufacturing guidelines as all other drugs produced in the U.S. as dictated by the FDA.
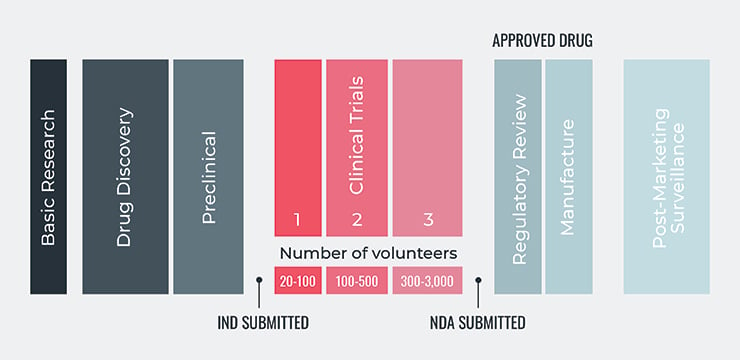
The five steps of the FDA's drug development process are:
- Discovery and Development: Research for a new drug begins in the laboratory. This can involve development of new chemical compounds, new insights about existing diseases and compounds, or development of new technologies needed for drug processing.
- Preclinical Research: Drugs undergo laboratory and animal testing to answer basic questions around safety.
- Clinical Research: Drugs are tested on people to make sure they are safe and effective.
- FDA Review: FDA review teams thoroughly examine all of the submitted data related to the drug or device and make a decision around whether or not to grant approval.
- FDA Post-Market Safety Monitoring: FDA monitors all drug and device safety once products are available for use by the public.
Manufacturing Transdermal Process
New drug development is an intense and lengthy process with numerous pitfalls. The complexity of manufacturing a drug for transdermal delivery is often underestimated. As a result, transdermal patches are particularly difficult to produce.
The transdermal patch is developed in a three-step process of mixing/blending, coating, and converting/packaging.
Mixing
In the mixing process, the adhesive is combined with the drug of choice and the necessary excipients. This involves a delicate balance of creating a homogenous and stable mixture without harming the integrity of the drug.
A drug formulation development service can aid in this process, as the mixing step requires knowledge of materials, properties of the desired product, and knowledge of the appropriate product equipment.
Coating
In the coating process, the mixture is carefully metered onto a polymer liner before being sealed in a convection oven. Knowledge of temperature, drug chemical behavior, and oven placement is essential during this step. An error in the sealing process can render the drug inactive.
Converting/Packaging
The converting/packaging stage appears the simplest, but it is often underestimated. Converting the laminate into multiple patches and then placing each patch in its pouch may not pose a direct risk to the medication, but it poses great risk to the delivery system itself.
Without trained engineers and equipment, thousands of patches can be lost during the final stage.
Chapter 4: Contract Development and Manufacturing Organizations for Transdermal Patches
Engaging With a CDMO
A contract development and manufacturing organization (CDMO) is a company that provides drug development and drug manufacturing services to other companies in the pharmaceutical industry.
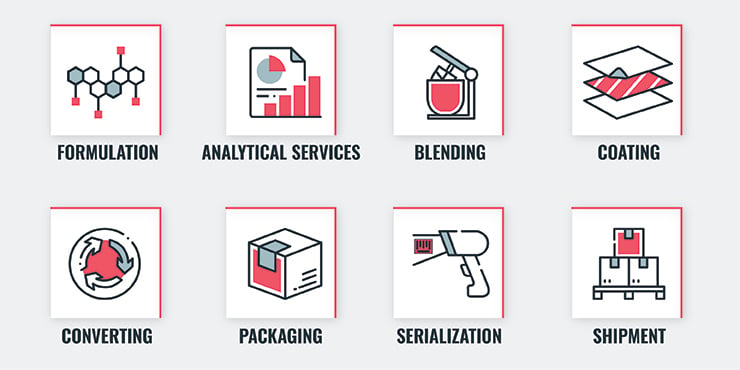
Full-service CDMOs take on nearly every aspect of drug development and manufacturing while organizing outsourcing on behalf of the client for additional services. Services provided by the CDMO include:
CDMOs are often referred to as CMOs, but it's important to note that these two types of organizations differ. While CMOs deal with pre-developed drugs, CDMOs are involved in the entire process — from development to manufacturing.
Why partner with a CDMO?
Drug development is expensive and time-consuming. Partnering with an experienced and acclaimed CDMO makes the process smoother through consistency, personalization, access to equipment, and scalability.
When you partner with a CDMO, you get access to all of their professionals, as well as all of their lessons learned. A strong CDMO has experience with every aspect of the process (research, formulation, FDA approvals, shipping, blending, serialization, etc.) and has likely handled a variety of hurdles during previous projects. As a result, they have accurate and efficient practices down to a science. Because transdermal patches make up a newer portion of the market, it can be extremely beneficial to work with a CDMO that has experience with your specific type of project.
Consistency From Pilot to Distribution
Even though CDMOs can outsource to other companies, a good CDMO has the experience and the facilities needed to control the entire process. These CDMOs have the end-to-end capability to handle all steps from initial drug concept to final marketing and distribution, which improves reliability and consistency.
Keeping all phases of the development and manufacturing process under one roof not only streamlines and expedites drug production, but it also helps avoid errors in communication across companies. And because CDMOs are often engaged with multiple market participants, they are also continually expanding their technological expertise and capabilities, which in turn benefits your project.
Personalized Service
Though all drugs are required to follow the same development guidelines, few will face the exact same developmental process.
A strong CDMO has a roster of professionals with diverse areas of expertise. All CDMOs need top researchers, chemists, and engineers, but a successful CDMO will also invest in development professionals, manufacturing experts, and distribution experts.
Transdermal patches require different equipment, materials, and packaging than traditional drug delivery systems. With the right team on board, a CDMO can tailor a development process based on speed to market, technical needs, personnel needs, and distribution obstacles.
Access to Equipment and Facilities
Consistency also requires access to reliable equipment and facilities. If the goal is to have one team follow the technical journey of a patch's development and manufacturing process, maintaining consistency is much simpler when all necessary equipment is in the same location and all team members have familiarity with that equipment. Therefore, a CDMO should have equipment on site, and said equipment should be well-regulated and repaired.
One of the key advantages to partnering with a CDMO is that a pharmaceutical company can avoid the costs of purchasing (or sometimes developing) necessary equipment. When seeking a CDMO, it's important to discuss equipment needs up front, as many pieces of transdermal patch manufacturing equipment are relatively new and not yet utilized within all CDMOs.
Scalability
Putting a new drug on the market is unpredictable, making new drug development a risky endeavor for pharmaceutical companies. As a result, private companies can face substantial losses or slowed production when faced with a slow or busy season.
By partnering with a CDMO, companies are able to scale more easily. The company doesn't have to take full ownership of its own production, and if a drug doesn't sell right away, financial resources can be more easily diverted to another product.
Partnering with a CDMO also allows for quick production during busy seasons without having to invest in additional equipment, ensuring that time to market continues to meet expectations.
Questions to Ask When Looking For a CDMO
A CDMO should fit the needs of your company and your products. Evaluate their technical expertise, equipment, and previous experience to determine if they can create and deliver your drug.
When looking for a CDMO, start by getting answers to the following questions:
- What delivery systems do they specialize in?
- What types of transdermal patches have they already developed/manufactured?
- Do they have the capacity to meet your project deadline?
- What equipment is readily available? Do they have backup parts and plans in the event of equipment failure?
- How many senior engineers and scientists will be dedicated to your specific project?
- Do they take a risk-based approach when analyzing data?
- How will they assess quality?
- Where do they invest their capital?
- Will they be able to see the project from development to manufacturing?
- If they outsource work, who do they outsource, and how are those companies vetted?
- Do they have a backlog in investigations?
- Will they conduct a lab-scale assessment prior to production to identify any issues?
- What will collaboration look like between your technical team and the CDMO's team?
- Do they share your security and safety priorities?
Most importantly, look at how a potential CDMO handles its partnerships. They are there to help the development and manufacturing process, not hinder it.
Partnering with a CDMO can put pharmaceutical companies at additional risk — the final product produced by the CDMO can either build or diminish your reputation. At the same time, partnering with the right CDMO can simplify your developmental and manufacturing processes and help you move on a faster timeline. By taking the time to evaluate your CDMO, you can help determine whether the partnership will be beneficial to the production of your specific project.