What You Need to Know About Adhering Dermal Patches
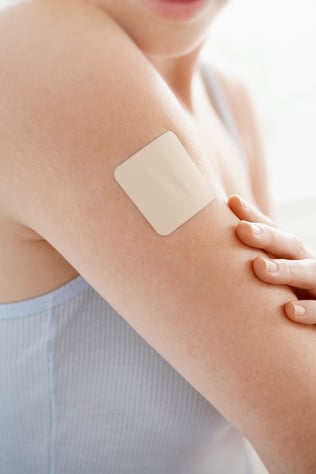
A transdermal drug delivery system (TDDS), or adhering dermal patch, is a medication delivery system that delivers a specific, controlled dose of a therapeutic drug across the skin and into the bloodstream.
Transdermal patch delivery systems have seen a slow but steady rise in prescription market share over the past 30 years. Drug manufacturers continue to progress the availability of dermal adhesive patch formulas to meet the demand for this safe, convenient, effective, and still emerging mode of drug administration. The transdermal drug patch market is expected to grow at a compound annual growth rate (CAGR) of 4.5% until 2023.
Benefits of Transdermal Adhesive Patches
This continued market growth of transdermal adhesive patches is reflective of the many benefits associated with its form and function, including:
- User-Friendly. Transdermal drug delivery systems provide continuous, steady levels of the active pharmaceutical ingredient (API) and can be immediately stopped. Less frequent dosing and improved patient compliance are key advantages in patient care when using an adhesive dermal patch.
- Fewer Side Effects. Drug delivery through dermal patch reduces gastrointestinal (GI) complications of drug administration and averts hepatic first-pass metabolism, allowing for improved therapeutic bioavailability.
- Painless. Freedom from painful intramuscular injections and patient ease of use further increase patient adherence and compliance to medication when using adhering patches as a drug delivery system.
- Non-Oral. Patients suffering from decreased swallowing capabilities (dysphagia) and other digestive issues are often prescribed adhesive patches as an alternative for safety and efficacy.
What to Look for in Adhesives
Drug properties must be considered when selecting an adhesive for a transdermal delivery patch. Currently, there is limited availability of commonly used medications in transdermal formulations. Drugs that require relatively consistent plasma levels are good candidates for transdermal drug delivery.
 Further, formulas with a low molecular weight (less than 1000 Da) and lipophilic and hydrophilic molecules that have a short half-life tend to be more easily formulated in a transdermal patch as they permeate the skin more efficiently.
Further, formulas with a low molecular weight (less than 1000 Da) and lipophilic and hydrophilic molecules that have a short half-life tend to be more easily formulated in a transdermal patch as they permeate the skin more efficiently.
Drug delivery, rate, flux, physical integrity of the finished product, skin maceration, and chemical stability are all variables that influence the transdermal transport and bioavailability of the API through the structural layers of the skin.
Skin in the Game
Skin adhesion is a critical measure in the design and manufacturing of transdermal patches in order to achieve intended drug delivery to the patient. Many available transdermal patches are meant to be removed within 24 hours of dermal application. However, new formulations have been designed for longer wear times of even up to seven continuous days.
Skin integrity and dermal adherence quality of the patch are dependent on age, race, and patient health which contribute to overall skin variations. Likewise, variations of skin surface energy and skin stretching at different body locations affect transdermal patch wear and performance. These factors must be considered by an adhesives expert in order to achieve a consistent delivery profile across a varied patient population.
Human skin creates a highly protective barrier. Skin biocompatibility is a major concern in dermal applications. Skin adhesives must be designed to include a biocompatible adhesive delivery system to prevent skin irritation.
Three Considerations for Skin Adhesions
An additional consideration in the formulation of a dermal adhesive patch is dependent upon its impact on skin, breathability, and adhesion efficacy—all of which are vital to the performance, efficacy, and safety of the system.
- Breathability. The adhesive’s ability to let skin breathe is essential to prevent skin maceration or breakdown from moisture exposure during use of a patch. Breathability depends on the moisture vapor transmission rate (MVTR) of the skin patch as well as the adhesive properties. In some dermal drug applications, maintaining a certain hydration level at skin and adhesive interface is critical for maximum drug flux. If the hydration levels are not ideal, the skin becomes weak, resulting in potential skin damage and pain during removal of the patch. Choosing the right adhesive is important in maintaining the integrity of the skin to avoid wounds, possible infection, and skin irritation.
- Irritation. Avoiding skin irritation is a common goal for transdermal applications. Patients can reduce skin irritation by applying the patch to different locations, so the same piece of skin is not in constant contact with the adhering dermal patch.
- Durability. The durability of the adhesive bond needs to accommodate physical activity and long wear scenarios. The durability of the adhesive will be tested against the friction of clothing, internal and external moisture, skin debris, and oil.
Adhesives for the future
With the correct adhesive, patch design, and care in API selection, the benefits of intravenous infusion can be closely duplicated by using skin as the system of entry. Patient compliance and continuity of care in drug administration are critical in improved patient health. Continued development and improvement of current TDD system adhesives allow a patient to better maintain steady drug levels and eliminate injections. These benefits and an improved side effect profile remove common barriers to drug administration. This ultimately improves patient compliance and outcomes.
As our current healthcare system undergoes change, there is a growing trend to popular patient-administered treatments via remote care settings. Continued improvements in adhesives will pave the way for future applications of transdermal dermal drug delivery patches that will help meet the increased demands of the new virtual and remote healthcare model.
